

He left research in 1918 to become Master of Trinity College. In 1897 he showed that cathode rays (radiation emitted when. However, an English physicist J.J Thomson started several different cathode ray experiments in order to find out the ration of the charge to the mass of the.
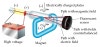
In addition to being awarded the Nobel Prize in 1906, he was knighted in 1908 by King Edward VII. Thomson managed to estimate its magnitude by performing experiments with charged particles in gases. How did Thomson determine that the cathode ray particles were a new particle and not simply an atom, molecule or ion carrying a negative charge. Thomson published 13 books and more than 200 papers in his lifetime. Cathode Ray Tube Experiment by J.J.Thomson Thomsons First CRT Experiment Thomsons Second CRT Experiment Thomsons Third CRT Experiment Experiment Summary. They had one daughter, Joan, and one son, George Paget Thomson, who went on to become a physicist and win a Nobel Prize of his own. Thomson married Rose Paget, one of his students, in 1890. this experiment was performed two months after Thomson first announced that cathode rays were very small, negatively charged particles. This was the first use of mass spectrometry. In doing so, he discovered that neon was composed of two different kinds of atoms, and proved the existence of isotopes in a stable element. This led to one of his other famous discoveries in 1912 when he channeled a stream of ionized neon through a magnetic and an electric field and used deflection techniques to measure the charge to mass ratio. In 1906, Thomson began studying positively charged ions, or positive rays.


 0 kommentar(er)
0 kommentar(er)
